Research Interests
Exploring the frontiers of molecular science and crystal engineering through innovative approaches and computational methods
Research Area 01
Exploring the concept of structural landscape in crystal engineering
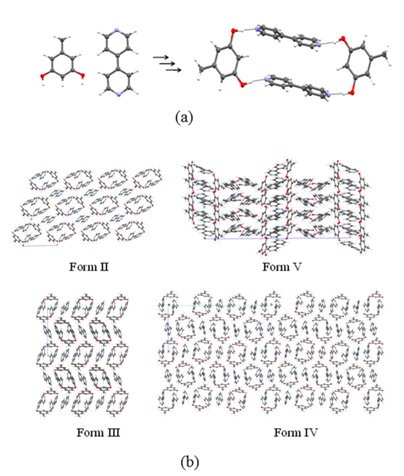
The structural landscape provides a holistic picture of small structural and energetic changes that take place during the late stages of crystallization of an organic compound. Any given compound is associated with a large number of putative crystal structures that lie in a small low energy window. Many of these structures are never accessed experimentally for reasons that are not fully clear. The landscape idea itself contains information about high energy crystalline forms, polymorphs, pseudopolymorphs, high Z’ cases which may hint about the probable molecular features based on the intermolecular interactions during the crystallization process. Studies of the crystal landscape could lead to an understanding of the kinetic pathways that control the crystallization processes. In general, the idea of crystal structure landscape collectively brings the essential ideas of modern crystal engineering: crystallization mechanism, high Z` crystal structures as well as polymorphism, and further emphasise the importance of kinetic features in the supramolecular synthesis. We have seen recently that the device of small chemical substitutions at innocuous positions of a molecule is an effective method of accessing crystal structures in the landscape of the subject molecule that cannot be so easily obtained experimentally. The device of solid solutions has been shown to be an even finer probe into the landscape.
📚 12 References
- Madhu, R.; Desiraju, G. R. IUCrJ, 2021, 8, 178-185
- Chakraborty, S; Joseph, S; Desiraju, GR. Angew. Chem. Int. Ed., 2018, 57, 9279-9283.
- Chakraborty, S; Desiraju, GR. CrystEngComm, 2018, 20, 2793-2805.
- Mukherjee, A; Dixit, K; Sarma, SP; Desiraju, GR. IUCrJ 2014, 1, 228-239
- Dubey, R; Pavan, MS; Guru Row TN; Desiraju, GR. IUCrJ 2014, 1, 8-18
- Mukherjee, A; Desiraju, GR. Cryst. Growth Des. 2014, 14, 1375-1385
- Dubey, R; Desiraju, GR. Chem. Commun. 2014, 50, 1181-1184
- Dubey, R; Desiraju, GR. Angew. Chem. Int. Ed. 2014, 53, 13178−13182.
- Dubey, R; Pavan, MS; Guru Row TN; Desiraju, GR. IUCrJ 2014, 1, 8-18
- Mukherjee, A; Desiraju, GR. Cryst. Growth Des. 2014, 14, 1375-1385
- Dubey, R; Desiraju, GR. Chem. Commun. 2014, 50, 1181-1184
- Dubey, R; Desiraju, GR. Angew. Chem. Int. Ed. 2014, 53, 13178−13182.
Research Area 02
Halogen bonding
📚 8 References
- Jain, H.; Sutradhar, D.; Roy, S.; Desiraju, G. R. Angew. Chem. Int. Ed., 2021, 60, 12841-12846.
- Desiraju, G. R. Acta Crystallogr. Sect. C 2019, 75, 1188–1189.
- Aakeroy,C. B.; Bryce, D. L.; Desiraju,G. R.; Frontera, A.; Legon, A. C; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; Resnati, G. Pure Appl. Chem. 2019, 91, 1889–1892.
- Saha, S; Rajput, L; Joseph, S; Mishra, MK; Ganguly, S; Desiraju, GR. CrystEngComm, 2015, 17, 1273-1290.
- Mukherjee, A; Desiraju, GR. IUCrJ 2014, 1, 49-60.
- Mukherjee, A; Tothadi, S; Desiraju, GR. Acc. Chem. Res. 2014, 47, 2514-2524.
- Tothadi, S, Desiraju, GR. Chem. Commun. 2013, 49, 7791-7793.
- Tothadi, S; Joseph, S; Desiraju, GR Cryst. Growth Des. 2013, 13, 3242-3254.
Research Area 03
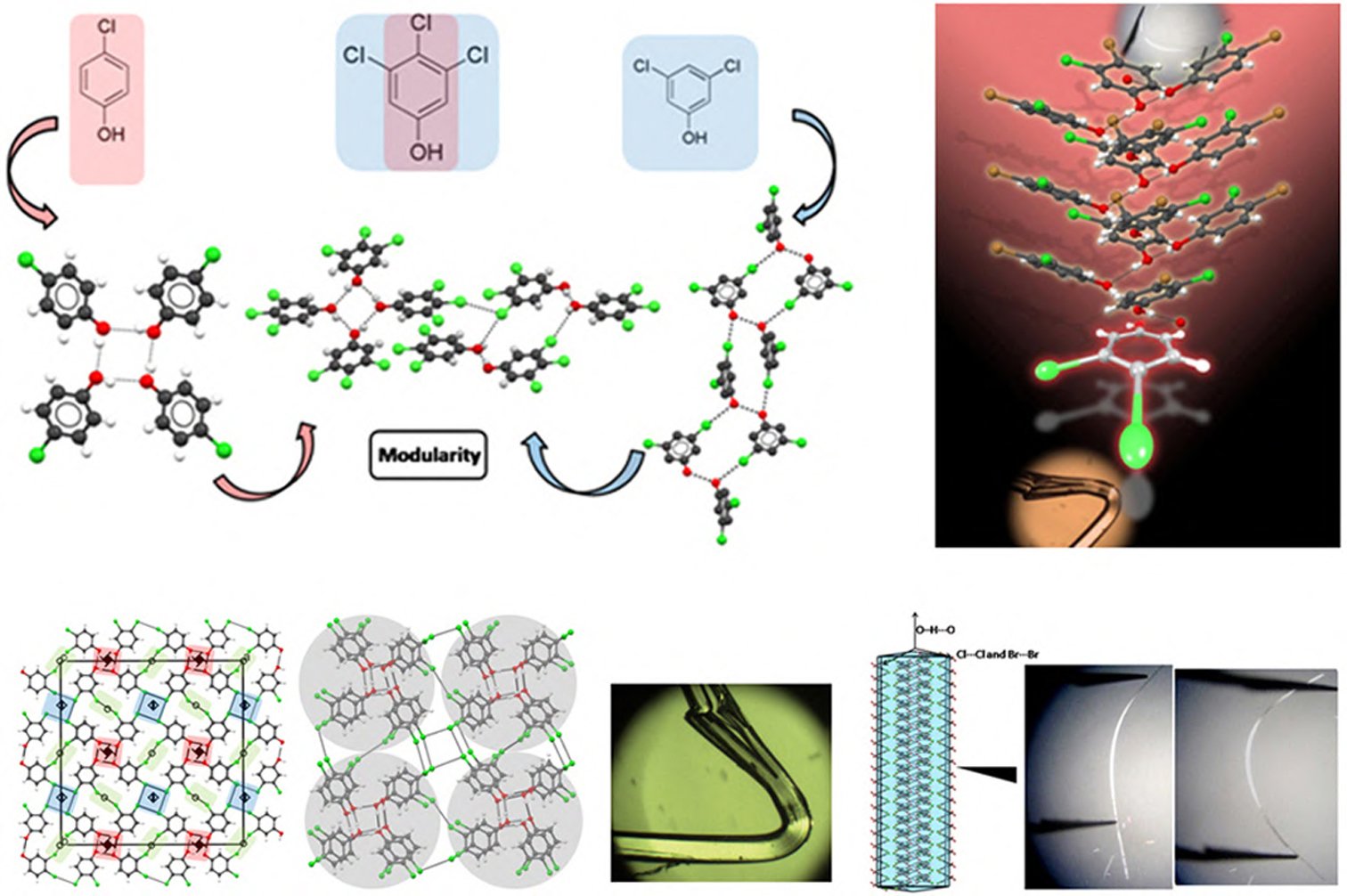
Synthesis of higher-order cocrystals
📚 7 References
- Roy S; Gaur R; Paul, M; Rajkumar, M and Desiraju G.R. Cryst. Growth Des. 2022, 22, 12, 7578-7589.
- Paul, M; Chakraborty, S; Desiraju, G. R. J. Am. Chem. Soc., 2018, 140, 2309-2315.
- Dubey, R.; Mir, N.A.; Desiraju, G. R. IUCrJ, 2016, 3, 102-107.
- Mir, N.A.; Dubey, R.; Desiraju, G. R. IUCrJ, 2016, 3, 96-101.
- Dubey, R.; Desiraju, G. R. Angew. Chem., Int. Ed. 2014, 53, 13178−13182.
- Chakraborty, S; Rajput, L; Desiraju, G. R. Cryst. Growth Des. 2014, 14, 2571-2577.
- Tothadi, S, Desiraju, G. R. Chem. Commun. 2013, 49, 7791-7793.
Research Area 04
Mechanical properties of molecular crystals
Nanoindentation is an effective method to assess the mechanical response of solids with high precision, and on extremely small volumes. It provides useful information on the mechanical properties of polymorphic drugs, which in turn allows for developing an understanding of their stability in the solid state. We use nanoindentation and nanoscratching as tools to study mechanical anisotropy, interaction characteristics, desolvation processes, polymorphism, phase stability, and molecular migration in molecular crystals. It may be used as a direct measure of molecular and crystal energies of molecular crystals. In all these studies we try to understand these phenomena as a function of crystal packing. As in all types of crystal engineering, an understanding of the intermolecular interactions can lead to property oriented crystal design, and we have shown that complex properties may be deliberately turned on or off in organic crystals: one essentially fine-tunes the degree of isotropy/anisotropy by modulating interactions such as hydrogen bonding, halogen bonding, π···π interactions, and C−H···π interactions.
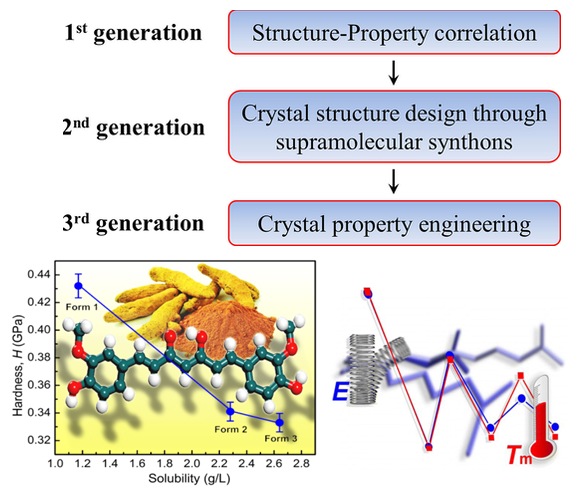
📚 7 References
- Saha, S.; Mishra, M. K.; Reddy, C. M.; Desiraju, G. R. Acc. Chem. Res. 2018, 51, 2957-2967.
- Saha, S; Desiraju, G. R. J. Am. Chem. Soc., 2017, 139, 1975−1983.
- Saha, S; Desiraju, G. R. Chem. Eur. J., 2017, 23, 4936−4943.
- Mishra, MK; Sanphui, P; Ramamurty, U; Desiraju, G. R. Cryst. Growth Des. 2014, 14, 3054-3061.
- Mishra, MK; Varughese, S; Ramamurty, U; Desiraju, G. R. J. Am. Chem. Soc. 2013, 135, 8121-8124.
- Varughese, S; Kiran, MSRN; Ramamurty, U; Desiraju, G. R. Angew. Chem., Int. Ed. 2013, 52, 2701- 2712.
- Mishra, MK; Desiraju, G. R.; Ramamurty, U; Bond, A Angew. Chem., Int. Ed. 2014, 53, 13102−13105.
Research Area 05
Supramolecular synthon identification by FT–IR spectroscopy
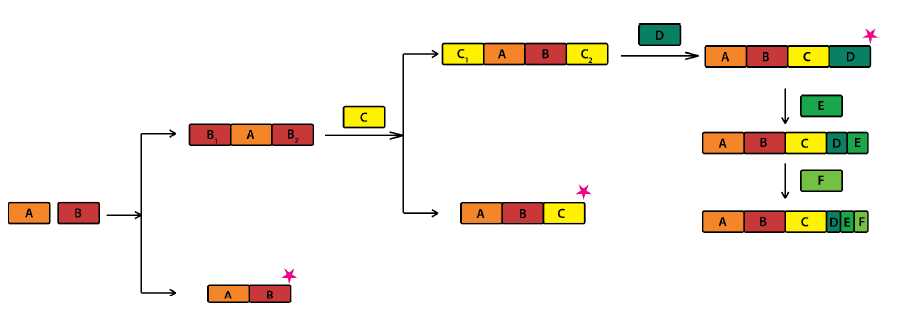
Supramolecular synthons, structural units that contain all geometrical and chemical information inherent to the phenomenon of primary recognition between functional groups, are of great significance in crystal engineering. Heterosynthons hold a position of special importance among them because they provide a predictive justification, in terms of unique intermolecular interactions, as to why two molecules should co-crystallize. As supramolecular synthons grow in significance and usefulness with the advent of high throughput crystallization and screening methods, the need is felt for their identification with a method that is simple, accurate and not as time, cost and labor-intensive as Single Crystal X-ray Diffraction (SCXRD) or Powder X-ray Diffraction (PXRD). When it comes to choosing a technique for synthon identification that is comparatively cheap, easy to use and ubiquitous in its availability, IR spectroscopy seems to be the natural choice. Using this concept we have developed an ‘IR-marker’ method for simultaneous identification of multiple H-bonded synthons and their transferability has also been probed. Coming to a more difficult case, we have been able to distinguish topological and geometrical variations, in the same halogen bonded synthon by means of a ‘Graded IR filter’. Recently, the IR characteristics for a variety of weak but frequently used C-H…X synthons have also been identified. We have developed a four-step strategy for identifying supramolecular synthons using IR spectroscopy, outlined in the scheme below:
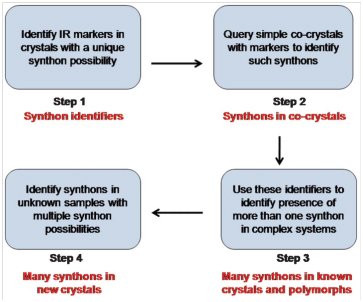
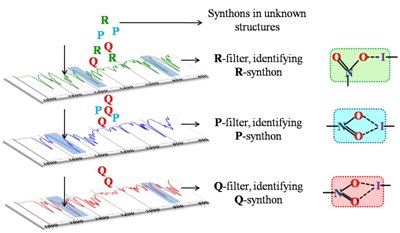
📚 4 References
- Saha, S; Rajput, L; Joseph, S; Mishra, MK; Ganguly, S; Desiraju, GR. CrystEngComm, 2015, 17, 1273-1290.
- Saha, S; Ganguly, S; Desiraju, GR. Aust. J. Chem. 2014, 67, 1840–1848.
- Chakraborty, S; Ganguly, S; Desiraju, GR. CrystEngComm 2014, 16, 4732-4741
- Mukherjee, A; Tothadi, S; Chakraborty, S; Ganguly, S; Desiraju, GR. CrystEngComm 2013, 15, 4640-4654.
Research Area 06
Polymorphs, salts and cocrystals of active pharmaceutical ingredients (APIs) and their physicochemical properties
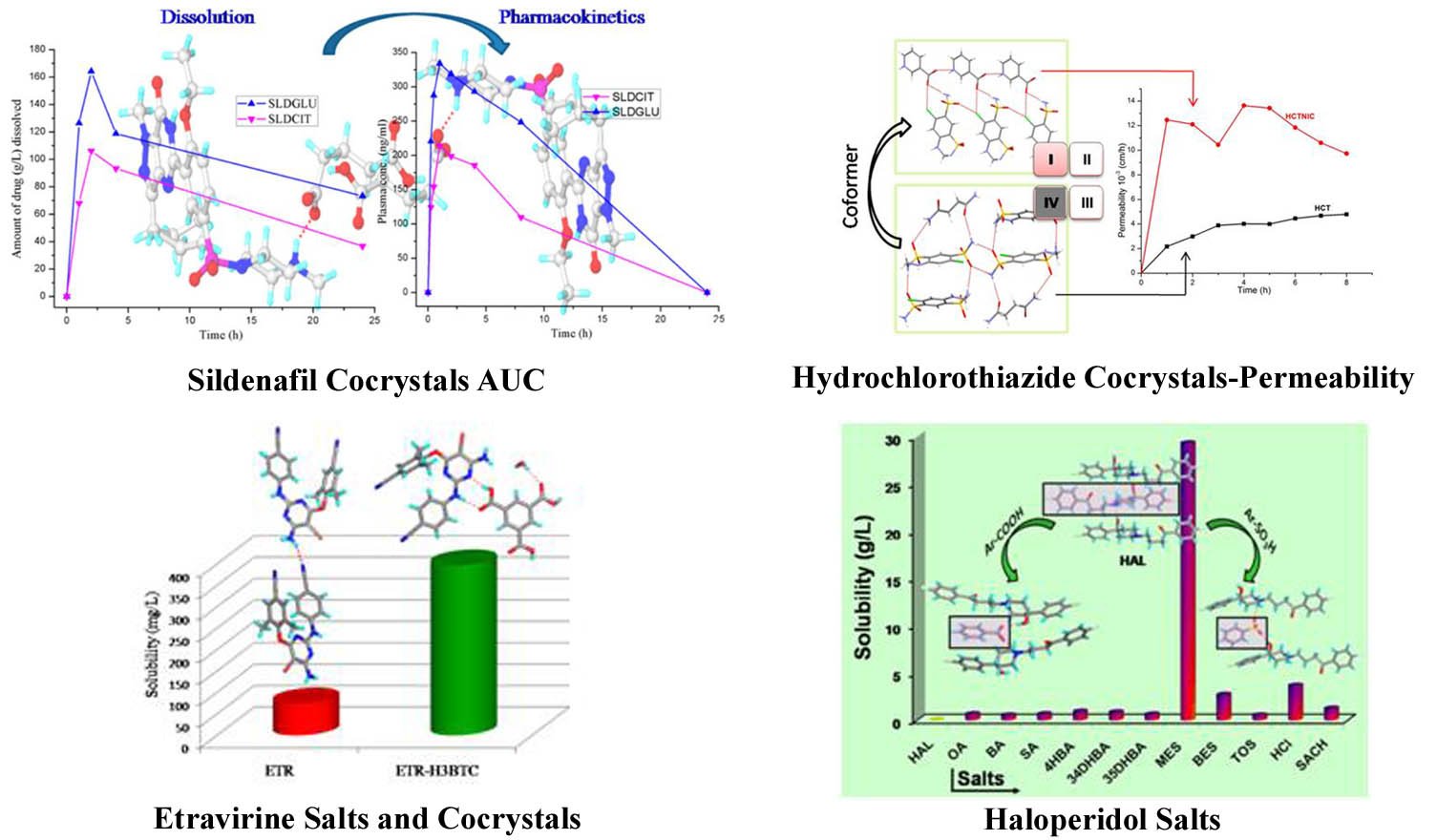
📚 9 References
- Nangia, AK; Desiraju, GR Angew Chem. Int. Ed. 2022, 61, e202207484
- Maity, DK; Paul, RK; Desiraju,GR Mol. Pharmaceutics, 2020, 17, 4435-4442
- Rajput, L; Banik, M; Yarava, JR; Joseph, S; Pandey, MK; Nishiyama, Y; Desiraju, GR IUCrJ, 2017, 4, 466-475.
- Samie, A; Desiraju, GR; Banik, M Cryst. Growth Des., 2017, 17, 2406–2417.
- Gopi, SP; Banik, M; Desiraju,GR Cryst. Growth Des., 2017, 17, 308–316.
- Gopi, SP; Ganguly, S; Desiraju, GR Mol. Pharmaceutics, 2016, 13, 3590–3594.
- Banik, M; Gopi, SP; Ganguly, S; Desiraju GR Cryst. Growth Des., 2016, 16, 5418–5428.
- Rajput, L.; Sanphui. P.; Desiraju, GR. Cryst. Growth Des. 2013, 13, 3681-3690.
- Sanphui, P; Rajput, L. Acta Crystallogr., Sect. B 2013, 70, 81-90.
📚 1 Indian Patent Filed in 2013
- Title of the invention: Sildenafil-cocrystals and salts and uses thereof; Assignee: Indian Institute of Science
List of PhD students from 1979 to 2018
- Jagarlapudi A. R. P. Sarma
- T. Siva Rama Krishna
- Veerapaneni Nalini
- K. V. Radha Kishan
- V. Rao Pedireddi
- Kumar Biradha
- C. V. Krishnamohan Sharma
- B. Satish Goud
- D. Shekhar Reddy
- Venkat R. Thalladi
- N. N. L. Madhavi
- Ram Thaimattam
- Ram K. R. Jetti
- Srinivasan K. Kuduva
- Sunil Panigrahi
- Aparna Vema
- Rahul Banerjee
- C. Malla Reddy
- Dinabandhu Das
- Basavoju Srinivas
- Prashant M. Bhatt
- Tejender Thakur
- Srinu Tothadi
- Arijit Mukherjee
- Ritesh Dubey
- Manish Mishra
- Subhankar Saha
- Shaunak Chakrabarty